英语周报 2018-2022 八年级 课标 35答案


【参考范文】One day, Spotty returned from his daily walk with a broken leg. Appearing exhausted, he came to myroom and sat near me, the leg bleeding. I called my mother and she quickly tied a bandage around his leg andgave him food to eat. I was very upset. But the next day, Spotty followed me wherever I went happily asusual though he limped a bit. After this incident my relation with Spotty became more intense. I reallyadmired him a lot for his courageAlmost a year later, one midnight we heard Spotty barking breathlessly. We rushed out and saw himbarking continuously, heading somewhere. After some time Spotty became quiet. I patted him on his backand came inside. The next morning, my heart skipped a beat when I didn't see Spotty. I searched for him ineach and every corner but in vain. And this time he had gone and would never come back. I cried and waitedfor him. But there were no signs of him and I only say him looking at me with his sparking eyes in my dream


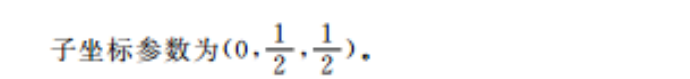
20.(14分)(1)1分)球形(1分)(2)小于(1分)Zn原子半径小,且价电子排布为全满结构,第一电离能更大(1分)(3)平面三角形(1分)(4)F>N>H(1分)sp3(1分)HF分子间能形成氢键(1分)(5)①1:1(2分)②2.06×19(2分,其他合理答案也给分)③(0,,)(2分)【解析】(1)基态F原子的价电子排布式为2s2p3,2其轨道表达式为回基态Zn原子的价电子排布式为3d04s2,占据最高能层电子的电子云轮廓图形状为球形。(2)K与Zn属于同一周期,Zn原子半径小,且价电子排布式为3d04s2的全满结构,故K的第一电离能小于Zn的第一电离能(3)CO中C原子的价层电子对数为3,孤电子对数为0,其立体构型为平面三角形(4)元素的非金属性越强,电负性越大,故NHHF2的组成元素的电负性由大到小的顺序为F>N>H;其中N原子的价层电子对数为4,杂化方式为sp2;HF分子间能形成氢键故能形成分子缔合体(HF)(5)①由图可知,ZnF2中Zn的配位数为6, KZnF3中Zn的配位数为6,二者之比为1:1②每个ZnF2晶胞中含有2个Zn2和4个F-,ZnF2晶体的密度为103×2gNA·(a×10-cm)2×(c×10-cm)2.06×102Na2g·cm-s③由晶胞结构图知,D点原子处于面心位置,D的原子坐标参数为(0,2·2)

以上就是英语周报 2018-2022 八年级 课标 35答案,更多英语周报答案请关注本网站。






