英语周报2018一2022第5朝答案


第二节One possible versionI asked him, "Why did you not tell my roommate that youare my brother?"He replied with a smile," If so, they willmake fun of you for having such a brother and you 'll surely feelembarrassed."Hearing his words I felt so touched that tears filledmy eyes. Then he took out some money from his pocket and put itin my hand, telling me to mind my health and not to worry abouthim. I pulled my brother into my arms and burst out crying. Thatyear, my brother was 20 years old and I was 23Several years later, when my brother got married, he wasasked who was the person he loved most. Without a secondthought, he answered, "My sister. " Then he continued, One day,my parents happened to be out and I became seriously ill. It wasmy sister who took me immediately to the hospital and finally Irecovered. From that day on, I was determined to do all that I couldfor my sister because I thought she was the dearest person to me.On such an occasion, I was moved to tears again by what he saidand took great pride in having such a nice brother

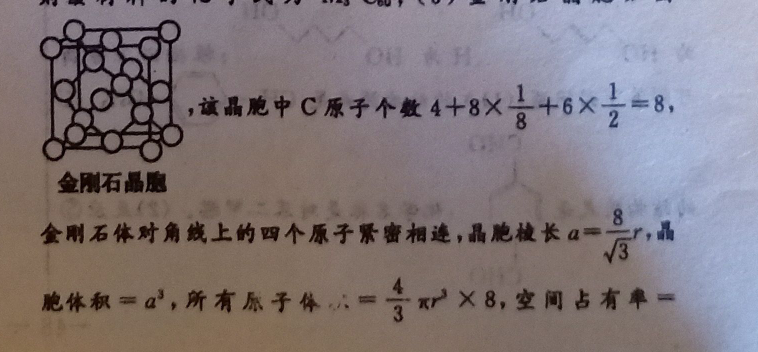
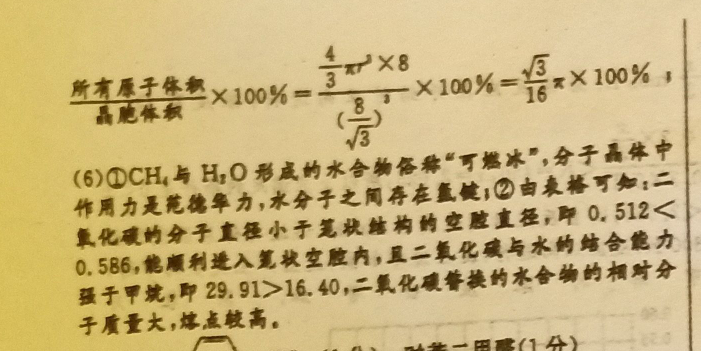
35.(1)无(1分)(2)sp2(1分)=(1分)(3)氧化石曼烯可与水形成氢键更稳定(2分)(4)12(2分)M3C0(2分)(5)3x×100%(2分)F(6)①氢键、范德华力(2分)②能(1分)二氧化碳替换的水合物(1分)解析:(1)图乙中,1号碳原子形成4个共价单键,所以其价层电子对个数是4,图乙中1号C与相邻C没有形成π键;(2)每个C原于要形成4个共价键,根据图知,每个C原子形成2个共价单健、1个共价双健,共价单健为σ键、共价双键中1个是0健、1个是π健;图甲中1号C的杂化方式为sp2。该C与相邻C形成的健角=120°;(3)氧化石县烯粒可与水分子形成氩健,而石墨烯不能,形成氫健使稳定性增强;(4)如图M原子位于晶胞的棱上与内部,梭上有12个M,内部有9个M,其个数为12×1+9=12,C分子位于顶点和面心,Cm分子的个数为8×g+6×2=4,M原子和Cm分子的个数比为311则该材料的化学式为MCo;(5)金刚石晶胞如图,晶胞中C原子个数4+8×1+6×=8金刚石晶息金刚石体对角线上的四个原子紧曹相迹,晶胞棱长a=8,画胞体积=a2,所有原子小=x×8,空间占有单=8所是本100%=100%=8x×100%(6)①CH4与H1O形成的水合物俗称“可燃冰”,分于晶体中作用力是范德华力,水分子之同存在篮健:②由袁格可知:二氧化碳的分子直小于笔状地构的空腔直径,P0.512<0.586,能顺利进入复秋空腔内,且二氧化碳与水的结合能力骚子甲就,即29.91>16.40,二氧化普换的水合物的相对分子质量大,熔点较高。

以上就是英语周报2018一2022第5朝答案,更多英语周报答案请关注本网站。






