2018-2022英语周报初一4答案


短文改错(共10小题;每小题1分,满分10分)Last Sunday, I went to Tian'anmen Square with my cousin, a boy of nineyear old. Because it was the first timehat he has come to beijineverything could interest him. He took many photos, saying they would behowing to his friends, most of who had never visited Beijing. Then a foreigner in the fifties caught our attentionTo my greatly surprise, before I could react, my cousin went up. With fluent English, he asked if she needed any helpKnowing her passport A missing, we immediately helped her got in touch with the police. What a kind boywas

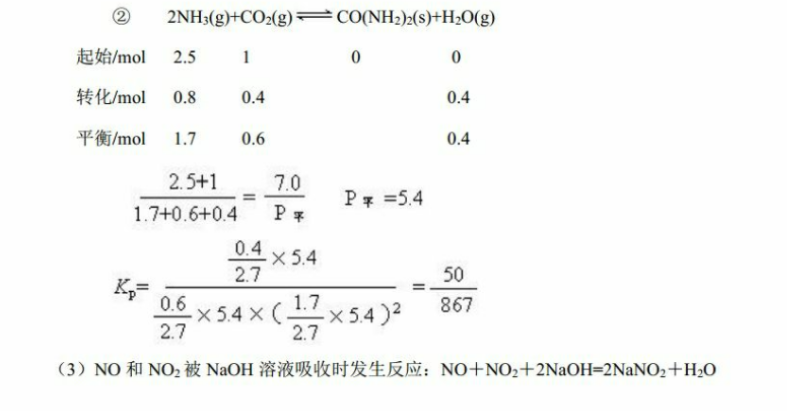

催化剂27.(1)4NH3(g)+6NO(g)5N2(g)+6H2O(g)△H=1808kJ·mo1(2分)(2)①02m0lL·min(1分):12.5(1分)②54(1分):B2(0058也可)(1分(3)11.2(1分):56(1分)(4)3:1(2分)(5)①阴极(1分)2H2O+NO-3e=NO3+4H(1分)②1.12(2分)解析:(1)△H=∑反应物键能一∑生成物键能=4×3×391+6×633-5×946-6×2464=1808kJ·moll。故该反应的热化学方程式为催化剂4NH3()+6N0(g)(g)+6H2O(g)△H=1808kJ·mol(2)O 2NH3(g)CO2(g)=CO(NH2)(s)+H O(g)起始/mol4转化/mol10.50.50.55min时/mo30.50.50.5v(NH3)=lmol/lL/5min=0.2 mol.L I. min由于CONH2)2是固体,计算气体总物质的量时不应计算在内,CO2的物质的量的分数100%=12.5%3+0.5+0.52 2NH3(gHCO2(g)+CO(NH2)2(s)+H20(g)起始/mol2.510转化/mol0.80.40.4平衡/mo1.70.60.42.5+17.01.7+0.6+04pPx=540.4542.7E2>×54X(1.70.627X54)2(3)NO和NO2被NaOH溶液吸收时发生反应:NO+NO2+2NaOH=2NaNO2+H2O过量的NO2被NaOH溶液吸收时发生反应:2NO2+2NaOH=NaNO3+NaNO2+H2On(NaOH)=3×10mol,又由于n(NaNO3yn(NaNO2)=12,故n(NaNO3)=1×10mol,n(NaNO2)=2×10-mol,故n(NO)=5×107tmol,n(NO2)=2.5×10mol,催化剂(4)6NO3+4xNH3(2x+3)N2+6H2O(NO和NO2用NO3表示)6.725.6x=1.25故NO和NO2的体积比为3:1(5)根据工作原理示意图,电解时A极NO得电子生成NH4,发生还原反应,故A极为阴极,电极反应式为3NO+15e-+18H=3NH4+3H2O:B极为阳极,电极反应式为10H2O+5NO=5NO3+20H:总反应式为8NO+7HO电=3NHNO+2HNO,为使电解产物全部转化为NHNO3,需补充物质NH3。标准状况下,每消耗448LNO,需要补充NH3体积为1.12L。

以上就是2018-2022英语周报初一4答案,更多英语周报答案请关注本网站。






