2022~2英语周报答案


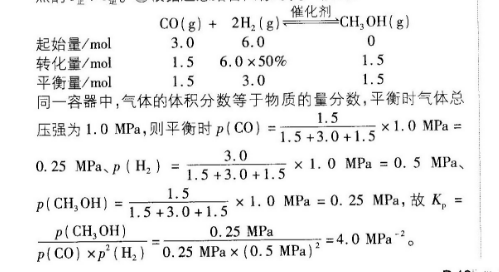

18.(11分,除标注外,每空1分)(1)CHOH()+O2(g)=CO2(g)+2H2O()△H=-726.6kJ·mol(2分)(2)①p1

第二节书面表达【写作思路】根据试题要求,考生需要写一篇记叙文,记述身边一位值得尊敬和爱戴的人。首先考生需要介绍自己最尊敬和爱戴的人是谁,简单地介绍一下他(她)。然后,陈述为什么他(她)值得尊敬和爱戴。最后,可以对前文进行一个总结,使文章结构严谨。本文的写作重点在"尊敬和爱戴的原因",考生可围绕这个要点适当拓展,从性格特点、处事风格等方面展开写作。【范文赏读】The most beloved and respected person around me is my teacher, Ms LiThough she has been teaching English for twenty years, she is still passionateabout teaching. She is kind and considerate towards us just like our dear motherWe all respect her because she always tries new ways to make her classes livelyand interesting. Hardworking and knowledgeable, she is one of the best teachersin our school. When we have a problem, we will turn to her for help. She alwaystalks to us patiently and helps us to find a solution. In our eyes, she is not onlyour teacher, but also our best friend. Those are why she deserves our respect【亮点词句】亮点词汇: beloved, respected, be passionate about, be consideratetowards, turn to sb. for help, in one's eyes亮点句式:Why引导的表语从句( why she deserves our respect)

以上就是2022~2英语周报答案,更多英语周报答案请关注本网站。






