2022英语周报课标提升高一答案

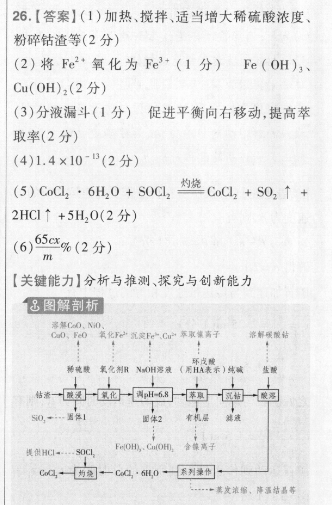

26.【答案】(1)加热、搅拌、适当增大稀硫酸浓度、粉碎钴渣等(2分)(2)将Fe2氧化为Fe2(1分)Fe(OH)3、Cu(OH)2(2分)(3)分液漏斗(1分)促进平衡向右移动,提高萃取率(2分)(4)1.4×10(2分)(5)C0C2·6H2O+SOC2杓CoC2+SO2↑+2HCI↑+5H2O(2分)(6)%(2分)【关键能力】分析与推测、探究与创新能力18图解剖析CaD、FeO氧化下e2沉淀Fe、C2取离子溶解碳酸钻稀硫駿氧化剂RNH液(用HA表示)纯碱盐酸一一面面面-面俄HC…80,0.CaHn合离子系列录作一其发浓缩,降温结晶等【解题思路】(2)钴渣中加人稀H2SO4后,FeO转化为Fe2,分析已知表中数据及流程可知,为了除去溶液中的Fe元素,需将Fe2氧化为Fe3+;加入氢氧化钠调节溶液pH=6.8,从已知表格信息知可将Fe3、Cu2“全部转化为Fe(OH)3、Cu(OH)2除去。(3)从杂质去向分析可知,萃取是除去镍离子,萃取需要用分液漏斗。根据平衡移动原理,提高镍离子萃取率的有效措施之一是加入适量的碱,中和生成的硫酸。(4)Kn(C0CO3)=c(Co2+)·c(CO3-)=1.4×10。(5)氢氧化亚钴难溶于水,加入sOCl2,其与水反应生成氯化氢,抑制氯化亚钴水解。(6)由滴定过程中发生反应的方程式CoCl2+2AgNO3=C0(NO3)2+2AgCl↓可知,w(CoCl2)=cx12501000225.00×130100%=65cx%

第二节书面表达Dear Henry,Im Li Hua from your English speaking class lastterm. I m writing to ask for your helpNow I am preparing to attend MUN next weckHowever,have some difficulty with concepts andexpressions of the current affairs and speech skills. Iwonder if I can borrow your Dictionary of englishMedia. which you presented in class. Furthermore.I amnot quite sure about how to make a convincing andpersuasive speech at MUN. Could you be so kind as to giveme some advice?I know you have a very busy schedule, but I'd be verygrateful if you could spare some time to give me face-to-acc instructions. Thank you for your kindnessI'm looking forward to your replyYoursLi Hua

以上就是2022英语周报课标提升高一答案,更多英语周报答案请关注本网站。






